University of Texas MD Anderson Cancer Center
The University of Texas PDX Development and Trial Center (UTPDTC) is composed of investigators in the University of Texas MD Anderson (UTMDACC) and The University of University of Texas Southwestern Medical Center (UTSW) and is focused on development and preclinical investigation of PDXs from non-small cell lung cancer (NSCLC), colorectal cancer (CRC), pancreatic cancer (PDAC), and triple negative breast cancer (TNBC) tumor subtypes, as well as patients with selected genomic alterations across other histologies.
The primary goal for UTPDTC investigators is to optimize personalized biomarker-based cancer therapy and identify effective targeted drugs based on the molecular characteristics of each tumor, by developing PDX trial strategies for preclinical testing of single agents and drug combinations.
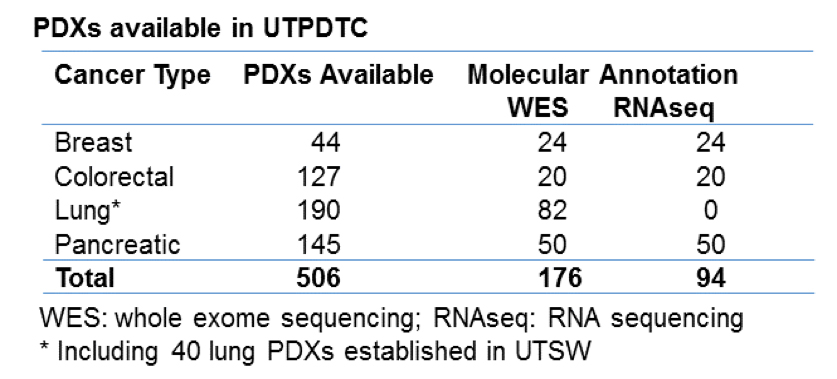
Over the past 9 years, we have established institution-wide efforts to generate novel PDX models and perform preclinical testing. Cumulatively the two institutions have hundreds of PDX models of different tumor types, including clinically-annotated models in non-small cell lung cancer (NSCLC, n=190 with 150 at UTMDACC and 40 at UTSW), colorectal cancer (CRC, n=127 models), pancreatic adenocarcinoma (PDAC, n=145 models), and triple negative breast cancer (TNBC, n=44 models). These models will allow the determination of the optimal treatments (single drugs or combinations) that should be tested in clinical trials in increasingly individualized, molecularly defined subsets of tumors. We have characterized many tumor subtypes in our existing PDXs and plan to characterize many more with the ultimate goal of developing drug combinations in defined tumor subsets in a context that can lead to clinical trials which will validate the experimental results.
The availability of hundreds of PDXs of diverse histologic types and molecular profiles provides a unique opportunity for synergistic interactions and collaborations among the UTPDTC investigators, PDXNet, ETCTN, and other investigators through PDXNet.
Publications:
- McAuliffe, P. F.; Evans, K. W.; Akcakanat, A.; Chen, K.; Zheng, X.; Zhao, H.; Eterovic, A. K.; Sangai, T.; Holder, A. M.; Sharma, C.; Chen, H.; Do, K. A.; Tarco, E.; Gagea, M.; Naff, K. A.; Sahin, A.; Multani, A. S.; Black, D. M.; Mittendorf, E. A.; Bedrosian, I.; Mills, G. B.; Gonzalez-Angulo, A. M.; Meric-Bernstam, F. Ability to Generate Patient-Derived Breast Cancer Xenografts Is Enhanced in Chemoresistant Disease and Predicts Poor Patient Outcomes. PLoS ONE [Electronic Resource] 2015, 10, e0136851.
- Hao, C.; Wang, L.; Peng, S.; Cao, M.; Li, H.; Hu, J.; Huang, X.; Liu, W.; Zhang, H.; Wu, S.; Pataer, A.; Heymach, J. V.; Eterovic, A. K.; Zhang, Q.; Shaw, K. R.; Chen, K.; Futreal, A.; Wang, M.; Hofstetter, W.; Mehran, R.; Rice, D.; Roth, J. A.; Sepesi, B.; Swisher, S. G.; Vaporciyan, A.; Walsh, G. L.; Johnson, F. M.; Fang, B. Gene mutations in primary tumors and corresponding patient-derived xenografts derived from non-small cell lung cancer. Cancer Letters 2015, 357, 179-185.
- Wang, L.; Zhang, R.; Fang, B. Patient-derived xenografts from lung cancer and their potential applictions. In Patient Drived Tumor Xenograft Models: Promise, Potential and Practice; Uthamanthil, R.; Tinkey, P. Eds.; Academic Press: London, 2016; pp 273-289.
- Kim, M. P.; Evans, D. B.; Wang, H.; Abbruzzese, J. L.; Fleming, J. B.; Gallick, G. E. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nature Protocols 2009, 4, 1670-1680.
- Kim, M. P.; Truty, M. J.; Choi, W.; Kang, Y.; Chopin-Lally, X.; Gallick, G. E.; Wang, H.; McConkey, D. J.; Hwang, R.; Logsdon, C.; Abbruzzesse, J.; Fleming, J. B. Molecular profiling of direct xenograft tumors established from human pancreatic adenocarcinoma after neoadjuvant therapy. Annals of Surgical Oncology 2012, 19 Suppl 3, S395-S403.
- Thomas, R. M.; Truty, M. J.; Kim, M.; Kang, Y.; Zhang, R.; Chatterjee, D.; Katz, M. H.; Fleming, J. B. The canary in the coal mine: the growth of patient-derived tumorgrafts in mice predicts clinical recurrence after surgical resection of pancreatic ductal adenocarcinoma. Annals of Surgical Oncology 2015, 22, 1884-1892.
- Kang, Y.; Zhang, R.; Suzuki, R.; Li, S. Q.; Roife, D.; Truty, M. J.; Chatterjee, D.; Thomas, R. M.; Cardwell, J.; Wang, Y.; Wang, H.; Katz, M. H.; Fleming, J. B. Two-dimensional culture of human pancreatic adenocarcinoma cells results in an irreversible transition from epithelial to mesenchymal phenotype. Laboratory Investigation 2015, 95, 207-222.
- Corcoran, R. B.; Atreya, C. E.; Falchook, G. S.; Kwak, E. L.; Ryan, D. P.; Bendell, J. C.; Hamid, O.; Messersmith, W. A.; Daud, A.; Kurzrock, R.; Pierobon, M.; Sun, P.; Cunninghan, E.; Little, S.; Orford, K.; Motwani, M.; Bai, Y.; Patel, K.; Venook, A. P.; Kopetz, S. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. Journal of Clinical Oncology 2015, Epub ahead of Print.
- Fan, F.; Bellister, S.; Lu, J.; Ye, X.; Boulbes, D. R.; Tozzi, F.; Sceusi, E.; Kopetz, S.; Tian, F.; Xia, L.; Zhou, Y.; Bhattacharya, R.; Ellis, L. M. The requirement for freshly isolated human colorectal cancer (CRC) cells in isolating CRC stem cells. British Journal of Cancer 2015, 112, 539-546.
- Kopetz, S.; Lemos, R.; Powis, G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clinical Cancer Research 2012, 18, 5160-5162.
The PDX models from the MD Anderson PDTC that are available for supplemental projects can be viewed in the attached spreadsheet. If you need any additional information regarding collaborating with MD Anderson PDTC for a supplemental project, please contact bfang@mdanderson.org
FUNDING
This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. 1U54CA224065-01.